In 2016, Yunnan Cancer Hospital( two other titles - The Third Affiliated Hospital of Kunming Medical University, Kunming Medical University School of Clinical Oncology) and Yunnan Provincial Key Laboratory on Lung Cancer were tested separately forSpace Quality Controlby the following three authoritative institutions: European Molecular Genetics Quality Network, National Center for Clinical Laboratories and National Health and Family Planning Commission Pathological Quality Control Center, including items like: EGFR mutation testing, BRAF mutation testing, KRASmutation testing, PIK3CA mutation testing, circle tumor DNA and T790M mutation testing. All the above mentioned testing successfully passed the testing.
With molecular classification is gradually complete, individualized cancer diagnosis and treatment based on molecular detection has became an important developing trend, how to do a better standardized genetic testing is highly valued by medical institutions at home and abroad. External Quality Assessment is processed with several laboratory analyzing the same masked test sample, and then external independent authority evaluating operation ability of a laboratory. It is a laboratory comparison aims to define the testing ability of a laboratory, and also an objective criterion for laboratory quality. Through the international and domestic inter-laboratory quality assessment, the evaluation ability of the testing institution was presented, and the quality and management level of the testing work was improved, what’s more, molecular detection ability was further standardized and improved.

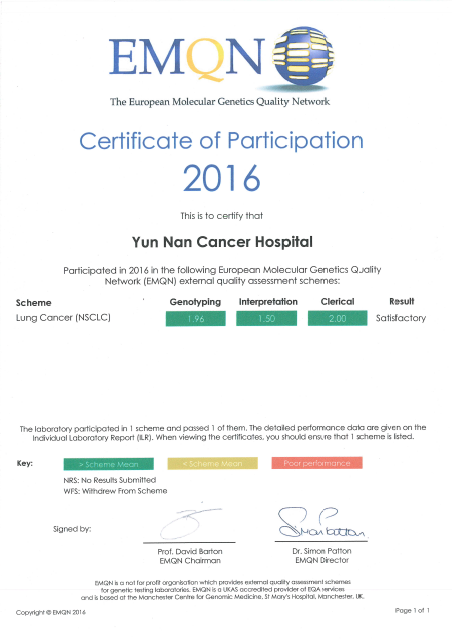
European Molecular Genetics Quality Network was established in 1998, and located in St. Mary's hospital in Manchester, British. It is a nonprofit institutions providing external quality assessment for global laboratories, and dedicates to improve the clinical molecular genetic testing quality. The molecular pathology quality control group of National Health and Family Planning Commission Pathological Quality Control Center was established in 2011, it commits to improve the molecular pathology detection capability.
Yunnan Cancer Hospital ( two other titles - The Third Affiliated Hospital of Kunming Medical University, Kunming Medical University School of Clinical Oncology) and Yunnan Provincial Key Laboratory on Lung Cancer passed the testing of the three institutions with full score, which means our laboratory possess a higher level of quality control in tissue sample and body fluid sample gene detection. It could provide an accurate molecular detection results for clinical departments and guide individualized diagnosis and treatment effectively.




